| Western blot (WB): | 1:500-2000 |
| Immunocytochemistry/Immunofluorescence (ICC/IF): | 1:50-400 |
| Flow Cytometry (Fixed): | 1:50-200 |
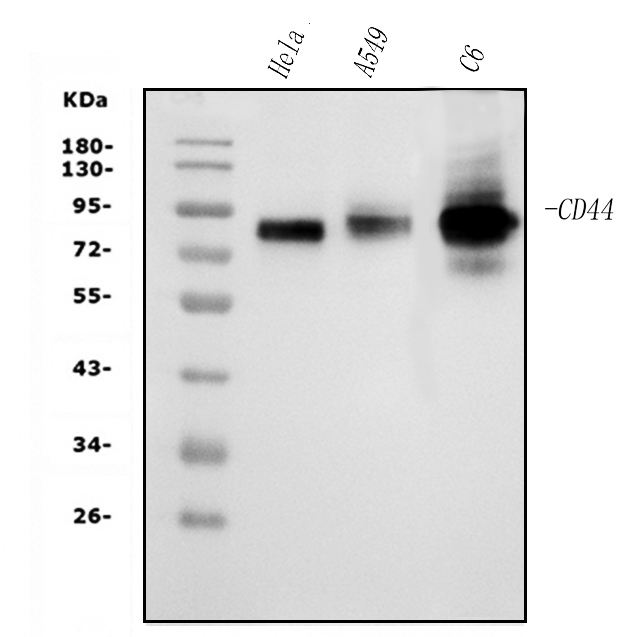
Figure 1. Western blot analysis of CD44 using anti-CD44 antibody (M00052-3). The sample well of each lane was loaded with 30 ug of sample under reducing conditions.
Lane 1: human Hela whole cell lysates,
Lane 2: human A549 whole cell lysates,
Lane 3: rat C6 whole cell lysates.
After electrophoresis, proteins were transferred to a membrane. Then the membrane was incubated with mouse anti-CD44 antigen affinity purified monoclonal antibody (M00052-3) at a dilution of 1:1000 and probed with a goat anti-mouse IgG-HRP secondary antibody (Catalog # BA1050). The signal is developed using ECL Plus Western Blotting Substrate (Catalog # AR1197). A specific band was detected for CD44 at approximately 82 kDa. The expected band size for CD44 is at 82 kDa.
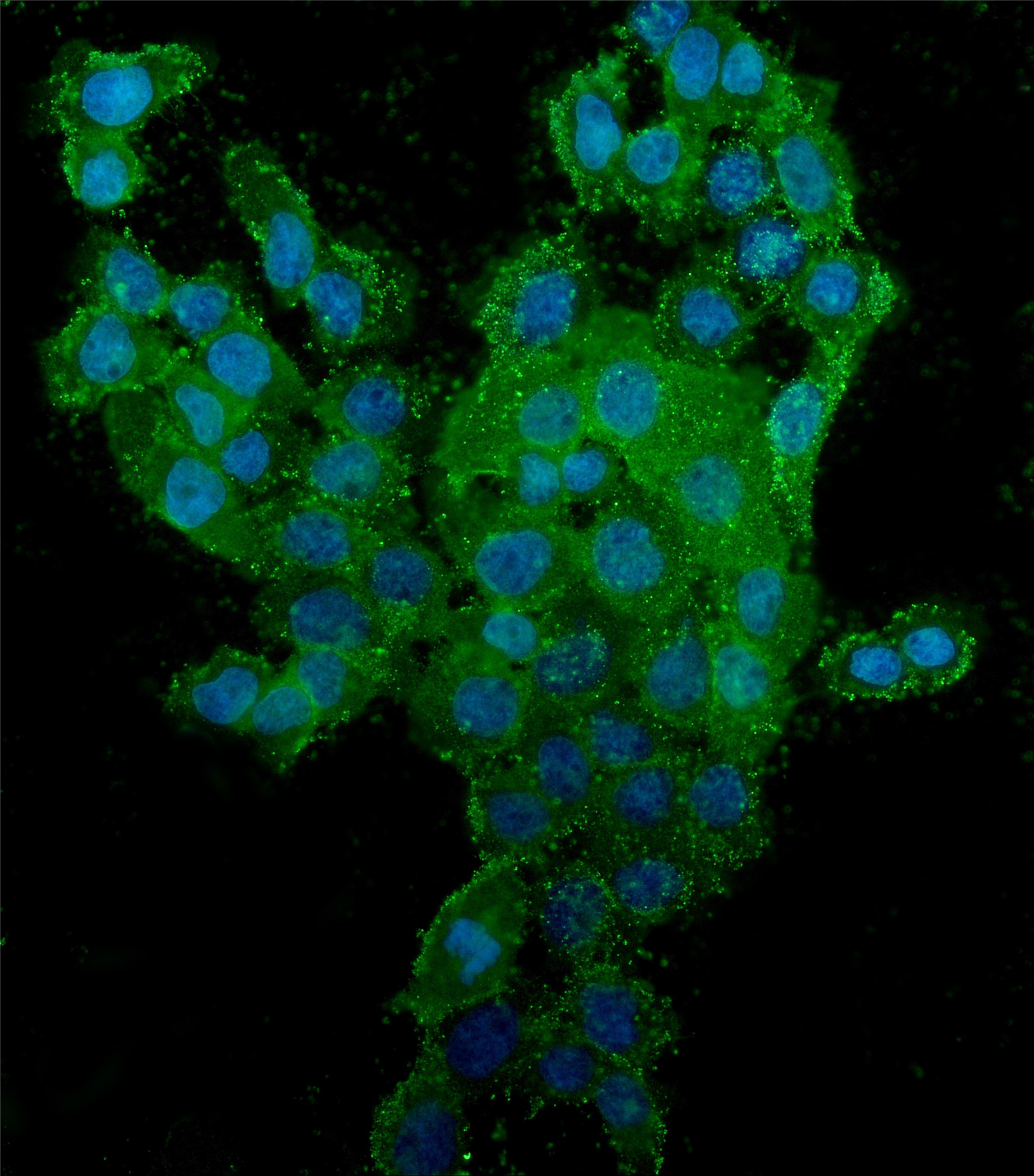
Figure 2. IF analysis of CD44 using anti-CD44 antibody (M00052-3).
CD44 was detected in an immunocytochemical section of A431 cells. The section was incubated with mouse anti-CD44 Antibody (M00052-3) at a dilution of 1:100. Dylight488-conjugated Anti-mouse IgG Secondary Antibody (green)(Catalog#BA1126) was used as secondary antibody. The section was counterstained with DAPI (Catalog # AR1176) (Blue).
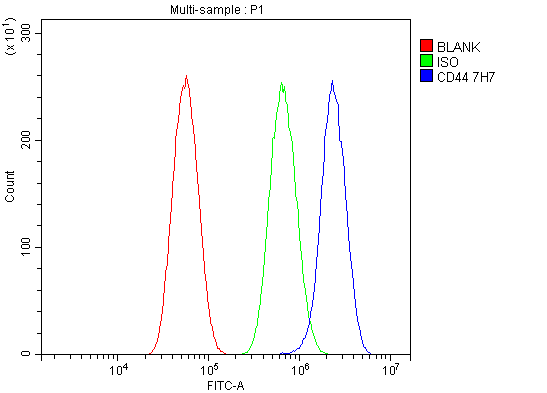
Figure 3. Flow Cytometry analysis of U87 cells using anti-CD44 antibody (M00052-3).
Overlay histogram showing U87 cells stained with M00052-3 (Blue line). The cells were fixed with 4% paraformaldehyde and blocked with 10% normal goat serum. And then incubated with mouse anti-CD44 Antibody (M00052-3) at 1:100 dilution for 30 min at 20°C. DyLight®488 conjugated goat anti-mouse IgG (BA1126) was used as secondary antibody at 1:100 dilution for 30 minutes at 20°C. Isotype control antibody (Green line) was mouse IgG at 1:100 dilution used under the same conditions. Unlabelled sample without incubation with primary antibody and secondary antibody (Red line) was used as a blank control.

Figure 1. Western blot analysis of CD44 using anti-CD44 antibody (M00052-3). The sample well of each lane was loaded with 30 ug of sample under reducing conditions.
Lane 1: human Hela whole cell lysates,
Lane 2: human A549 whole cell lysates,
Lane 3: rat C6 whole cell lysates.
After electrophoresis, proteins were transferred to a membrane. Then the membrane was incubated with mouse anti-CD44 antigen affinity purified monoclonal antibody (M00052-3) at a dilution of 1:1000 and probed with a goat anti-mouse IgG-HRP secondary antibody (Catalog # BA1050). The signal is developed using ECL Plus Western Blotting Substrate (Catalog # AR1197). A specific band was detected for CD44 at approximately 82 kDa. The expected band size for CD44 is at 82 kDa.

Figure 2. IF analysis of CD44 using anti-CD44 antibody (M00052-3).
CD44 was detected in an immunocytochemical section of A431 cells. The section was incubated with mouse anti-CD44 Antibody (M00052-3) at a dilution of 1:100. Dylight488-conjugated Anti-mouse IgG Secondary Antibody (green)(Catalog#BA1126) was used as secondary antibody. The section was counterstained with DAPI (Catalog # AR1176) (Blue).

Figure 3. Flow Cytometry analysis of U87 cells using anti-CD44 antibody (M00052-3).
Overlay histogram showing U87 cells stained with M00052-3 (Blue line). The cells were fixed with 4% paraformaldehyde and blocked with 10% normal goat serum. And then incubated with mouse anti-CD44 Antibody (M00052-3) at 1:100 dilution for 30 min at 20°C. DyLight®488 conjugated goat anti-mouse IgG (BA1126) was used as secondary antibody at 1:100 dilution for 30 minutes at 20°C. Isotype control antibody (Green line) was mouse IgG at 1:100 dilution used under the same conditions. Unlabelled sample without incubation with primary antibody and secondary antibody (Red line) was used as a blank control.


